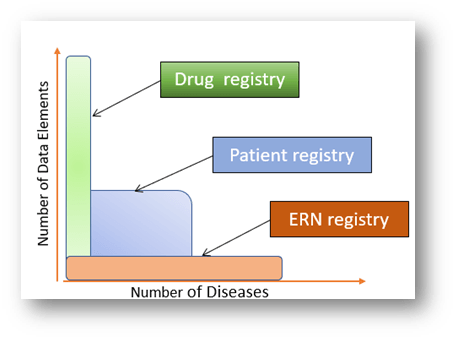
ERNs were founded on the principle that many rare disease issues are pan-European, and any single Member State cannot solve them alone. ERNs represent a unique Rare Disease innovation by the European Community to standardize and improve the treatment and research of patients with rare diseases across Europe.
In 2016 George Reynolds, our Managing Partner managed the tender process for the ICT platform for the 24 European Reference Networks. This resulted in two of his clients jointly winning the €5M contract to deliver the platform to the European Community. This game changing system was deployed in 300 hospitals throughout Europe.
Unfortunately, and perhaps inevitably since 2016, ERNs have become much more prescriptive and burocratic. Their day-to-day functioning are severely hampered by many challenges, including, lack of legal status as well as insufficient and unsustainable funding.
The paper “European Reference Networks: challenges and opportunities” provides further background. This was published by several ERN Coordinators in 2021. The paper expresses their frustration with the slow pace of ERN progress. Click button below to reveiw